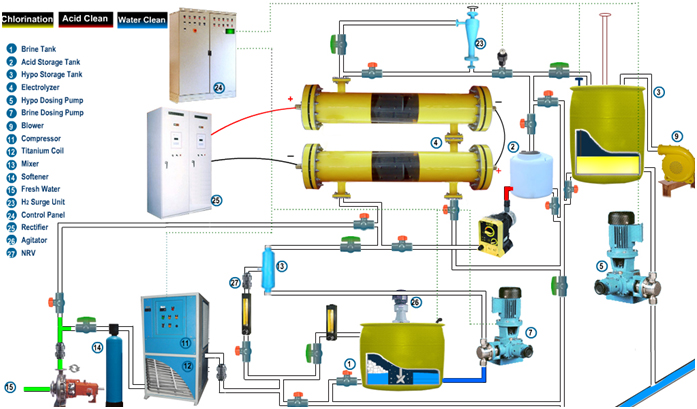
Process Description
The chlorination system produces sodium Hypochlorite solution by electrolysis process of synthetic Brine Solution. The process involves the following:
Water chilling
Brine Preparation
Electric conversion from AC Power to DC Power
Brine electrolysis process
Hydrogen dilution blower
Hypo chlorite-dosing system
Acid cleaning system
Water chilling:
The fresh feed water having suspended solids of size less than 0.5 mm (15 – 25 °C) and total hardness less than 50 PPM as Calcium Carbonate is fed into the package inlet at 3 bar (g) at Pressure and a flow rate of required LPH. A portion of this fresh soft water is fed to a chilling unit to reduce the temperature by 10 to 12o C.
Brine Preparation, filtration and feed system:
A portion of the fresh soft water is used for the preparation of saturated Brine Solution. Common crystal salt (98% purity and above) is added in the brine tank to prepare a saturated brine solution. The saturated brine solution is injected by means of brine forwarding pumps on to a static mixer. Here the saturated brine solution and raw water are mixed in a specific ratio to maintain the electrolyte concentration. This static mixer has been provided to facilitate the proper mixing of the raw water and the saturated brine solution. The dilute brine solution is fed to the Electrochemical Cell.
Electric Conversion from AC Power to DC Power
A Dedicated Transformer - Rectifier is provided to feed the Electrochemical Cell with Direct Current. This rectifier will be combined with transformer as a single unit. Necessary cables will be provided to transfer the current from the Transformer – Rectifier to the Electrochemical Cell.
Brine Electrolysis Process:
The Electro Chlorination System will consist of required streams each having multiple Electrochemical Cells with a capacity to produce required Kg/Hr of available chlorine as NaOCl. The following Electrochemical reactions will take place inside the Electrochemical Cell:
At the Anode: Chloride ions in the water deplete and Chlorine gets evolved
2 Cl → Cl2 ↑ + 2e
At the Cathode: Hydrogen and Hydroxyl ions evolve. These Hydroxyl ions react with Sodium atoms and form Sodium Hydroxide
2 H2O + 2e → 2OH- + H2 ↑
These Chlorine and sodium hydroxide react to give Sodium Hypo chlorite.
Overall Reaction: 2NaOH + Cl2 → NaOCl + NaCl + H2 ↑
Hypo Storage System
The Sodium Hypochlorite solution coming out from the cell along with Hydrogen gas is piped to a hypo storage tank cum degassing tank.
Hypo Dosing System:
The Hypochlorite solution is pumped to dosing area by Dosing Pumps
ACID CLEANING SYSTEM required for BRINE & SEA WATER ELECTRO CHLORINATION SYSTEM
The scaling formed by the side reactions of the Electrolysis process after long periods of operation (say approx. 3 to 4 weeks) get accumulated on the Electrodes present inside the Electrochemical Cell. Scaling has to be removed periodically by dissolving them in a solution of dilute Hydrochloric acid (5 % concentration). Dilute solution of Hydrochloric acid will be circulated inside the Electrochemical Cell for cleaning purpose. The acid cleaning system consists of an acid preparation tank and one acid-circulation pumps. The pump suction and discharge nozzles will be duly piped to the Acid storage tank and the Electrochemical Cell respectively for carrying out the cleaning process. At any point of time, the acid cleaning system will be used to clean cells in one stream only as the acid holding tank capacity will be designed for the required volume of acid only. Hydraulic Eductor devices are employed in the acid cleaning system to facilitate the transfer of concentrated acid and Caustic Soda to the dilute acid preparation tank. As the Concentrated HCl is used to clean the Electrochemical cells, the Caustic Soda is used to neutralize the spent acid in the dilute acid preparation tank. After the neutralization of spent acid, the same may be pumped out to waste water disposal path provided by the Client. To monitor the pH of the spent acid during this operation, a portable Ph meter will be provided by us to measure the pH value.
Note: Electro Chlorination Systems using TUBULAR TYPE ELECTRODES do not require Acid Cleaning System
