Chlorine Dioxide Chemistry
Chlorine dioxide (ClO2) is a neutral compound of chlorine in the +IV oxidation state.
It disinfects by oxidation; however, it does not chlorinate.
It is a relatively small, volatile, and highly energetic molecule, and a free radical even while in dilute aqueous solutions. At high concentrations, it reacts violently with reducing agents. However, it is stable in dilute solution in a closed container in the absence of light (AWWA, 1990).
Chlorine dioxide functions as a highly selective oxidant due to its unique, one-electron transfer mechanism where it is reduced to chlorite (ClO2-) (Hoehn et al., 1996).
The pKa for the chlorite ion, chlorous acid equilibrium, is extremely low at pH 1.8. This is remarkably different from the hypochlorous acid/hypochlorite base ion pair equilibrium found near neutrality, and indicates the chlorite ion will exist as the dominant species in water
The Oxidation Reduction reactions are as under;
CLO2(aq) + e- = CLO2-
CLO2- + 2H2O + 4e- = CL- + 4OH-
CLO3- + H2O + 2e- = CLO2- + 2OH-
CLO3- + 2H+ + e- = CLO2 + H2O
Physical Properties of ClO2
Highly soluble in water
In contrast to the hydrolysis of chlorine gas in water, chlorine dioxide in water does not hydrolyze to any appreciable extent but remains in solution as a dissolved gas.
It is approximately 10 times more soluble than chlorine (above 11°C), while it is extremely volatile and can be easily removed from dilute aqueous solutions with minimal aeration or recarbonation with carbon dioxide.
Chlorine dioxide cannot be compressed or stored commercially as a gas because it is explosive under pressure. Therefore, it is never shipped. Chlorine dioxide is considered explosive at higher concentrations which exceed 10 percent by volume in air, and its ignition temperature is about 130°C (266°F) at partial pressures (National Safety Council Data Sheet 525 – ClO2, 1967).
ClO2 Generation
Chlorine dioxide can be formed by sodium chlorite reacting with gaseous chlorine (Cl2(g)), hypochlorous acid (HOCl), or hydrochloric acid (HCl).
2NaCLO2 + CL2(g) = 2CLO2(g) + 2NaCL
2NaCLO2 + HOCL = 2CLO2(g) + NaCL + NaOH
5NaCLO2 + 4HCL = 4CLO2(g) + 5NaCL + 2H2O
| Generator Type | Reactants, byproducts, key reactions and chemistry notes | Special Attributes |
|---|---|---|
|
ACID-CHLORITE: |
4HCL + 5NaCLO2 → 4CLO2(aq) + CLO3- Low pH CLO3- possible Slow reaction rates |
Chemical feed pump interlocks required. Production limit ~ 25-30 lb/day. Maximum yield at ~ 80% efficiency. |
|
AQUEOUS CHLORINE CHLORITE: |
CL2 + H2O → [HOCL/HCL] [HOCL/HCL] + NaCLO2 → CLO2(g) + H/OCL- + NaOH + CLO3- Low pH CLO3- possible Relatively slow reaction rates |
Excess CL2 or acid to neutralize NaOH. Production rates limited to ~ 1000 lb/day. High conversion but yield only 80-92% More corrosive effluent due to low pH (~2.8-3.5). Three chemical systems pump HCL, hypochlorite, chlorite, and dilution water to reaction chamber. |
|
GASEOUS CHLORINE CHLORITE: |
CL2(g) + NaCLO2(aq) → CLO2(aq) Neutral pH Rapid reaction Potential scaling in reactor under vacuum due to hardness of feedstock. |
Production rates 5-120,000 lb/day. Ejector-based, with no pumps. Motive water is dilution water. Near neutral pH effluent. No excess CL2. Turndown rated at 5-10X with yield of 95-99%. Less than 2% excess CL2. Highly calibrated flow meters with min. line pressure ~ 40 psig needed. |
Proposals
Sodium Chlorite Solution-31% + HCL – 33% (2 Chemical based system)
Chlorine Gas + Sodium Chlorite Solution (25%)
Base
25 Kg/hr Capacity – 2 nos. (1W + 1 S)
Required dose: - 2 ppm of ClO2
Residual ClO2 – 0.8 ppm
Option 1:- Conventional ClO2 Generator
(Chlorine – Chlorite Method)
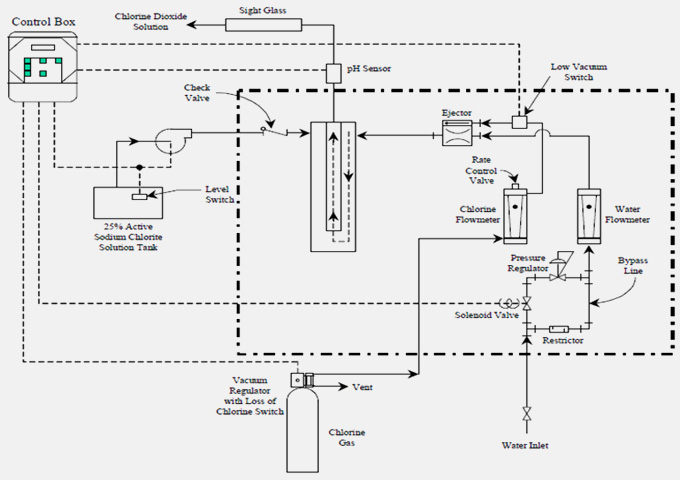
Base: - 25 Kg/hr Capacity of ClO2 generation
Chemicals (input) requirement:-
NaClO2 (25% Conc.) – 134 Kg/hr
or
NaClO2 (31% Conc.) – 108.12 Kg/hr
Chlorine gas – 13.15 Kg/hr
Major Components
Chlorinator (Chlorine gas based vacuum feed chlorinator)
Chlorine gas handling system
Sodium Chlorite Storage and handling system
ClO2 generator
ClO2 feeing piping and dispersion equipment’s
Capital Cost (in Rs.) - Tentative
Recurring Cost (in Rs.) - Tentative
